Use of Agricultural Residues to Remove Iron from Groundwater in Modified Airlift Aerator DOI: 10.32526/ennrj.17.3.2019.23
Main Article Content
Abstract
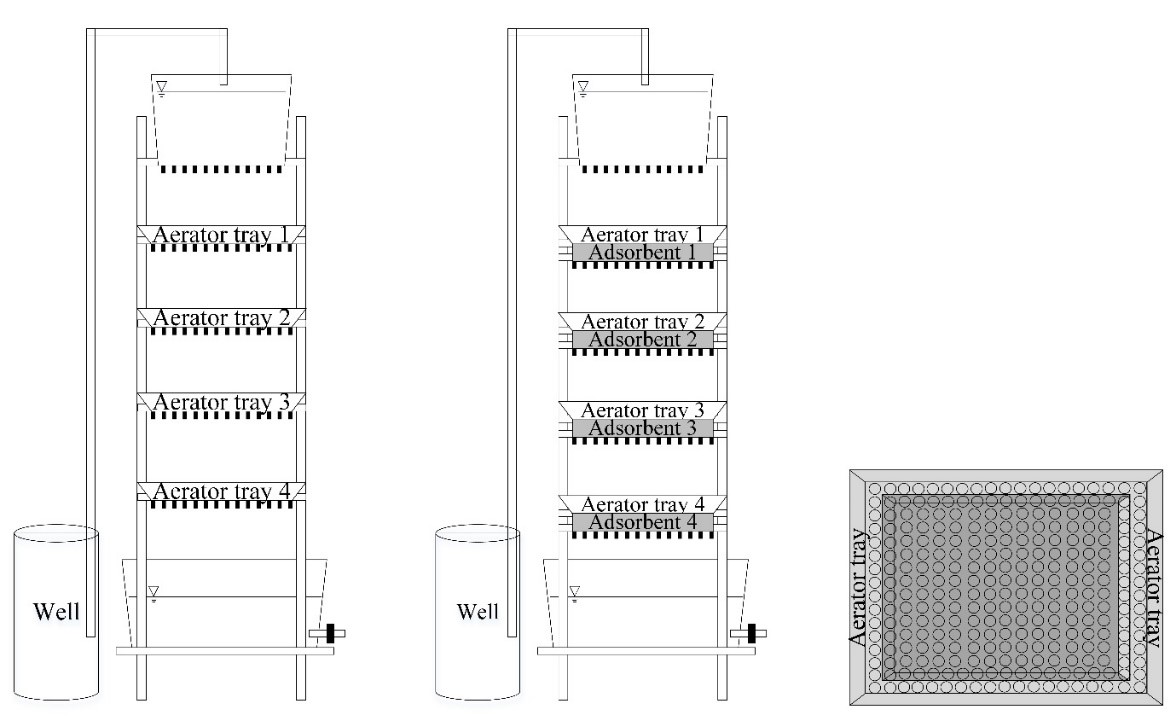
This work investigated groundwater iron adsorption capacity from rice husk, rice straw, water hyacinth and coconut shell, agricultural residues commonly found in Thailand. This study also investigated the adsorption behavior using an appropriate isotherm model in the batch process. The process was conducted using a modified airlift tray aerator. The use of a single adsorbent plate in a modified aerator obtained a removal capacity in the range of 0.3 to 0.9 mg/L, but the final iron concentration in the sample was above the regulatory standard. To increase the efficiency using the equivalent condition, the multiple adsorbent plate system was tested. The application of four rice husk plates achieved the allowance value and resulted in a final iron concentration of 0.28 mg/L. Based on the results, iron was reduced by increasing the number of adsorbent plates. Hence, rice husk can be sustainably used to adsorb iron in groundwater. At equilibrium, the adsorption isotherm was fitted to the Freundlich equation with an R2 value of 0.9805. This implied that the adsorption sites on the rice husk surface are heterogeneous in nature and presented a strong interaction between iron and rice husk. They revealed a maximum adsorption capacity of 0.73 mg/g. Moreover, this practice also decreased the amount of total hardness which could help alleviate nuisance and public health problems.
Article Details
Published articles are under the copyright of the Environment and Natural Resources Journal effective when the article is accepted for publication thus granting Environment and Natural Resources Journal all rights for the work so that both parties may be protected from the consequences of unauthorized use. Partially or totally publication of an article elsewhere is possible only after the consent from the editors.
References
2. Bhattacharya A, Kumar P. Water hyacinth as a potential biofuel crop. Electronic Journal of Environmental, Agricultural and Food Chemistry 2010;9(1):112-22.
3. Chai X, Li D, Cao X, Zhang Y, Mu J, Lu W, Zhao M. ROS-mediated iron overload injures the hematopoiesis of bone marrow by damaging hematopoietic stem/progenitor cells in mice. Scientific Reports 2015;5:10181.
4. Chaturvedi S, Dave PN. Removal of iron for safe drinking water. Desalination 2012;303:1-11.
5. Dalai C, Jha R. Rice husk and sugarcane baggase based activated carbon for iron and manganese removal. Aquatic Procedia 2015;4:1126-33.
6. Freundlich H. Uber die adsorption in losungen. Zeitschrift Fur Physikalische Chemie 1906;54:385-470.
7. Gorde SP, Jadhav MV. Assessment of water quality parameters: a review. Journal of Engineering Research and Applications 2013;3(6):2029-35.
8. Hoyos-Sánchez MC, Córdoba-Pacheco AC, Rodríguez-Herrera LF, Uribe-Kaffure R. Removal of Cd (II) from aqueous media by adsorption onto chemically and thermally treated rice Husk. Journal of Chemistry 2017;2017:1-8.
9. Ibrahim MM, El-Zawawy WK, Jüttke Y, Koschella A, Heinze T. Cellulose and microcrystalline cellulose from rice straw and banana plant waste: preparation and characterization. Cellulose 2013;20(5):2403-16.
10. Istirokhatun T, Rokhati N, Rachmawaty R, Meriyani M, Priyanto S, Susanto H. Cellulose isolation from tropical water hyacinth for membrane preparation. Procedia Environmental Sciences 2015;23:274-81.
11. Johar N, Ahmad I, Dufresne A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Industrial Crops and Products 2012;37(1):93-9.
12. Kasman M, Ibrahim S, Salmariza. Removal of iron from aqeous solution by rice husk: isotherm and kinetic study. Jurnal Litbang Industri 2012;2(2):63-70.
13. Kumar U, Bandyopadhyay M. Sorption of cadmium from aqueous solution using pretreated rice husk. Bioresource Technology 2006;97(1):104-9.
14. Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. Journal of the American Chemical Society 1918;40(9):1361-403.
15. Li WC, Law FY, Chan YHM. Biosorption studies on copper (II) and cadmium (II) using pretreated rice straw and rice husk. Environmental Science and Pollution Research 2017;24(10):8903-15.
16. Liyanage CD, Pieris M. A physico-chemical analysis of coconut shell powder. Procedia Chemistry 2015; 16:222-8.
17. Marsidi N, Abu Hasan H, Sheikh Abdullah SR. A review of biological aerated filters for iron and manganese ions removal in water treatment. Journal of Water Process Engineering 2018;23:1-12.
18. Mohan D, Singh KP, Singh VK. Trivalent Cr removal from wastewater using low cost activated carbon derived from agricultural waste material and activated carbon fabric cloth. Journal of Harzard Material 2006; B135:280-95.
19. Mohanty K, Jha M, Meikap BC, Biswas MN. Biosorption of Cr(VI) from aqueous solutions by Eichhornia crassipes. Chemical Engineering Journal 2006;117(1): 71-7.
20. Mohd Din AT, Hameed BH, Ahmad AL. Batch adsorption of phenol onto physiochemical-activated coconut shell. Journal of Hazardous Materials 2009;161(2-3):1522-9.
21. Mora Alvarez NM, Pastrana JM, Lagos Y, Lozada JJ. Evaluation of mercury (Hg2+) adsorption capacity using exhausted coffee waste. Sustainable Chemistry and Pharmacy 2018;10:60-70.
22. Nam H, Choi W, Genuino DA, Capareda SC. Development of rice straw activated carbon and its utilizations. Journal of Environmental Chemical Engineering 2018;6(4):5221-9.
23. Nasri-Nasrabadi B, Behzad T, Bagheri R. Extraction and characterization of rice straw cellulose nanofibers by an optimized chemomechanical method. Journal of Applied Polymer Science 2014;131(7):1-7.
24. Ndazi BS, Nyahumwa C, Tesha J. Chemical and thermal stability of rice husks against alkali treatment. BioResources 2008;3(4):1267-77.
25. Nikiforova TE, Kozlov VA. Regularities of the effects of the nature of polysaccharide materials on distribution of heavy metal ions in a heterophase biosorbent-water solution system. Protection of Metals and Physical Chemistry of Surfaces 2016;52(3):399-424.
26. Ogata F, Nakamura T, Kawasaki N. Adsorption capability of virgin and calcined wheat bran for molybdenum present in aqueous solution and elucidating the adsorption mechanism by adsorption isotherms, kinetics, and regeneration. Journal of Environmental Chemical Engineering 2018;6(4):4459-66.
27. Ooi K, Sonoda A, Makita Y, Torimura M. Comparative study on phosphate adsorption by inorganic and organic adsorbents from a diluted solution. Journal of Environmental Chemical Engineering 2017;5(4):3181-9.
28. Öztürk N, Kavak D. Boron removal from aqueous solutions by batch adsorption onto cerium oxide using full factorial design. Desalination 2008;223(1-3):106-12.
29. Pandey A, Soccol CR, Nigam P, Soccol VT, Vandenberghe LPS, Mohan R. Biotechnological potential of agro-industrial residues. II : cassava bagasse. Bioresource Technology 2000;74(1):81-7.
30. Pollution Control Department (PCD). Drinking water quality surveillance-drinking water quality standard [Internet]. 2018 [cited 2018 Oct 2]. Available from: https://www.pcd.go.th/info_serv/reg_std_water01.html#s3.
31. Rungrodnimitchai S. Modification of rice straw for heavy metal ion adsorbents by microwave heating. Macromolecular Symposia 2010;295(1):100-6.
32. Samsuri AW, Sadegh-Zadeh F, Seh-Bardan BJ. Adsorption of As(III) and As(V) by Fe coated biochars and biochars produced from empty fruit bunch and rice husk. Journal of Environmental Chemical Engineering 2013;1(4):981-8.
33. Santasnachok C, Kurniawan W, Hinode H. The use of synthesized zeolites from power plant rice husk ash obtained from Thailand as adsorbent for cadmium contamination removal from zinc mining. Journal of Environmental Chemical Engineering 2015;3(3):2115-26.
34. Santos M, Souza A, Silva C, Almeida R. Bioethanol production from coconut husk fiber. Ciência Rural 2016;46(10):1872-7.
35. Shafiquzzaman M. Removal of manganese from groundwater using a biological arsenic removal ceramic filter. Journal of Environmental Chemical Engineering 2017;5(2):1618-27.
36. Tekerlekopoulou AG, Pavlou S, Vayenas DV. Removal of ammonium, iron and manganese from potable water in biofiltration units: a review. Journal of Chemical Technology and Biotechnology 2013;88(5):751-73.
37. Uddin N, Islam T, Das S. A novel biosorbent, water-hyacinth, uptaking methylene blue from aqueous solution: kinetics and equilibrium studies. International Journal of Chemical Engineering 2014; 2014:1-13.
38. World Health Organization (WHO). Iron in drinking water. In: Guidelines for Drinking-Water Quality (Volume 2 - Health criteria and other supporting information). 2nd ed. Geneva: World Health Organization; 1996.
39. Zhang Y, Zhao J, Jiang Z, Shan D, Lu Y. Biosorption of Fe (II) and Mn (II) ions from aqueous solution by rice husk ash. BioMed Research International 2014;2014:1-10.
40. Zhao X, Zeng X, Qin Y, Li X, Zhu T, Tang X. An experimental and theoretical study of the adsorption removal of toluene and chlorobenzene on coconut shell derived carbon. Chemosphere 2018;206:285-92.