Submissions
Submission Preparation Checklist
As part of the submission process, authors are required to check off their submission's compliance with all of the following items, and submissions may be returned to authors that do not adhere to these guidelines.- This research manuscript has never been published or submitted to other journal(s) or other book(s) e.g. Proceedings, except for the Thai Journal of Public Health. Also, all authors consent to publishing this research manuscript in the Thai Journal of Public Health, Faculty of Public Health, Mahidol University.
- The manuscript should be prepared according to the instructions provided below
Author Guidelines
1.1 Manuscript Templates
1.2 Author Agreement Form
1.3 Article Types
1.4 Preparing a Manuscript
1.5 Peer Review Process (includes Manuscript Processing Flowchart)
1.6 Ethical Guidelines for Authors
1.7 Advertising and Marketing Policies
1.8 Appeals
Updated 2-September 2022
1.1 Manuscript Templates
Authors must prepare and submit their work on the appropriate manuscript template available from the links below. These template now comply with the journal's publishable format, including the required font type and sizes. The templates use DB Helvethaica X V3.2 (55 reg) font which can be downloaded here THJPH font (download, right click and select install).
Manuscript Template Original Research (English)
Manuscript Template Review (English)
1.2 Author Agreement Form
The corresponding author and other authors listed on the cover page must sign the Author Agreement Form, to indicate that all authors have approved the contents of the submitted manuscript and accept the journal’s copyright policy, and confirm that the work is not under review for publication elsewhere or has been published elsewhere.
1.3 Article Types
We invite the submission of unpublished original research and review articles which meet the Journal Aim and Scope. All submissions should be prepared according to the instructions provided below.
1.3.1 Research Articles
The justification for the research and research question must be clearly identifiable in the introduction. The materials and methods section must describe the materials, equipment and research procedures with sufficient detail and accuracy. The results section must demonstrate appropriate analysis, presentation and interpretation of research results, and results presented in tables/figures must be correct and clear. In the discussion section, authors must provide sufficient evidence-based reasons for their findings, and outline the limitations and strengths of their research, in addition to drawing comparisons with previous findings in this field. The conclusion must be appropriate and must indicate the public health implications of the findings and provide suggestion for future research.
1.3.2 Review Articles
- Narrative reviews must demonstrate that they are novel and necessary, provide a thorough overview and critique of an area of research, identify gaps in research knowledge, and assist in shaping new research questions. They must also be appropriate and balanced (not one-sided), cite original references, summarize information correctly, and include sufficient critical evaluation of the studies cited.
- Systematic reviews should follow appropriate guidelines. The PRISMA guidelines (http://www.prisma-statement.org/) include a checklist and an example of a flow diagram used to show the article selection process; these documents contain information that is relevant to all types of systematic review. Authors may also refer to MOOSE guidelines for systematic reviews of observational studies, and ENTREQ guidelines for systematic reviews of qualitative studies. Systematic reviews must be novel and necessary, and must include sound rationale, address a clear question, clearly state the outcome(s) of interest, and demonstrate a comprehensive search for articles. A methods section should state the search terms and describe techniques (name article sources, such as bibliographic databases, and article evaluation criteria, including description of how article quality was assessed). A results section should cover the numbers of studies screened, included and excluded (including reasons for exclusion), and explicitly state the characteristics of studies. A discussion section should incorporate a summary of the evidence and its relevance to key groups, along with discussion of the limitations of the review. A conclusion section should include an interpretation of the findings, and suggestions for future research.
1.4 Preparing a manuscript
1.4.1 Language
Submissions be written in English.
1.4.2 Length
English language manuscripts: 2,500-3,500 words; These word counts exclude the title page, abstract and references. Manuscripts can have up to 5 tables or figures.
1.4.3 Style
- Title and authors' details
The manuscript title must identify and cover the main content of the article in a concise way (aim for no more than 15 words). Superscript numbers should be used after each author name to show their affiliated address. The name and contact details (postal address and email) of the corresponding author should be shown.
- Abstracts
Abstracts should be unstructured and must not contain references. Abstracts should state the research objectives, methods, results, conclusions and recommendations. Manuscripts written in English must have a standard abstract written in English (250 words max.), which contains more detail than a standard abstract and therefore improves the chances of international citation. The extended abstract must include an ethical approval statement (showing protocol approval number, name of ethics committee and date of approval) for research involving human subjects.
- Keywords
Keywords (3 to 5) should be written underneath each abstract. The most effective keywords allow published articles to be easily discovered, therefore, keywords should be chosen carefully to clearly reflect the important content of the article. Choose terms which frequently appear in the abstract and main text.
- Tables and figures
Tables and figures must be written in English and on separate pages. Tables and figures should be numbered consecutively using Arabic numerals. Both should be easily understandable without reading the main text, and must be mentioned at least once in brackets in the main text, for example: (Table 1),… (Figure 1).
Tables: Only a top rule, rule below the heading and bottom rule must be used. Column and row labels must be brief and clear and show units of measurement. A column referring to sample number and percentage should use the label n (%). For percentage distribution, authors should use one decimal point. For measures of average and data dispersion, such as mean and standard deviation, authors should use also use a single column Mean (SD), and use 2 decimal points. If footnotes are used, superscript Roman letters which link with the footnotes should be consecutively applied (e.g., a, b, c). Asterisks (*, **, etc.) should not be used in footnotes, as these are solely for denoting the level of statistical significance.
2. Figures: Figures include graphs/charts, diagrams and photographs or other images. Graphs/charts must be drawn using a computer program, such as MS Excel, GraphPad, etc.
Probability values should be shown with a lower-case p (italics), for example p < 0.05.
- Author declarations
Authors must declare author contributions, acknowledgements, source of funding, and conflicts of interest at the end of their manuscript. An ethical approval statement (showing protocol approval number, name of ethics committee and date of approval) for research involving human subjects must also be included. If applicable, clinical trial registration numbers or animal research approvals should also be stated at the end of the manuscript. Further guidance for the completion of these statements is provided under the Publication Ethics tab and in the downloadable templates (links above). Authors should also scan and upload a copy of the ethical approval certificate or letter on to the manuscript submission system, for work that involved human subjects. Likewise, authors should upload evidence of approval by an animal research committee for work that involved animals.
1.4.4 References
All references must be written in English and Vancouver referencing style. Number references in superscript in the order cited in the text. General claims can be supported using general sources (e.g., a review), but specific claims need to be supported by specific sources (e.g., research article(s)). References should only refer to respected sources, and unpublished data should not be cited. References must be verified by the author(s) against the original documents. For articles printed in a language other than English, indicate the language in parentheses after the article title. If a cited publication has more than 6 authors, list the first 6 authors followed by “et al.”. The title of a journal should be abbreviated according to the List of Journals Indexed in Index Medicus / NLM NIH. Telescope page numbers, e.g.,1: 125-9, e.g.,2: 181-95, should be used.
Examples of Referencing Style:
Journal article
Srichan W, Uruwan Y, Kijboonchoo K, Thasanasuwan W. A comparison of bioelectrical impedance analysis with deuterium dilution technique for body fat assessment in school-age children. Journal of Public Health 2014; 44 (3): 223-36. (In Thai)
Lalaeng T, Vatanasomboon P, Satheannoppakao W. Effects of health education program for changing snack consumption behavior among grade 5 students. Journal of Health Education 2019; 42(2): 12-22. (In Thai)
Reininger B, Mecca LP, Stine KM, Schultz K, Ling L, Halpern D. A type 2 diabetes prevention website for African Americans, Caucasians, and Mexican Americans: formative evaluation. JMIR Res Protoc 2013; 2(2): e24.
Abbass-Dick J, Xie F, Koroluk J, AlcockBrillinger S, Huizinga J, Newport A, et al. The development and piloting of an eHealth breastfeeding resource targeting fathers and partners as co-parents. Midwifery 2017; 50: 139-47.
Pangkanon S, Sawasdivorn S, Kuptanon C, Chotigeat U, Vandepitte W. Establishing of a national birth defects registry in Thailand. J Med Assoc Thai 2014; 97(6): S182-8.
McNulty B, Pentieva K, Marshall B, Ward M, Molloy AM, Scott JM, et al. Women's compliance with current folic acid recommendations and achievement of optimal vitamin status for preventing neural tube defects. Hum Reprod 2011; 26(6): 1530-6.
LaBrosse L, Albrecht JA. Pilot intervention with adolescents to increase knowledge and consumption of folate-rich foods based on the Health Belief Model. Int J Consum Stud 2013; 37(3): 271-8.
Murphy BL, Dipietro NA. Impact of a pharmacist-directed educational program on the long-term knowledge and use of folic acid among college women: a 12-month follow-up study. Pharm Pract (Granada) 2012; 10(2): 105-9.
Book and other monographs
APHA. Standard methods for the examination of water and wastewater. 21st ed. Washington, D.C.: APHA-AWWA-WEF; 2005
Bernstein M, Luggen AS. Nutrition for the older adult. Sudburry: Jones and Bartlett Publishers; 2010.
Gibson RS. Principles of nutritional assessment. New York: Oxford University Press; 2005.
Akepalakorn W, editor. The report of the Thailand National Health Examination Survey IV 2008-2009: Child's health. Nonthaburi: The Graphico Systems; 2011. (In Thai)
Chapter in book
Bradley C. Measuring quality of life in diabetes. In: Marshall SM, Home PD, Rizza RA, eds. The diabetes annual 10. Amsterdam: Elsevier Science; 1996. p. 207-24.
Agency publication
World Health Organization. Ecosystems and human well-being: Health synthesis. A report of the millennium ecosystem assessment. Geneva: WHO; 2005.
Thesis
Panjai P. Food sanitation situation and influencing factors in Phitsanulok municipality. [M.Sc. Thesis in Environmental Sanitation]. Bangkok: Faculty of Graduate Studies, Mahidol University; 2014.
Website
Ministry of Public Health, Department of Health, Thailand. Red-cheeked Thai women. Available from: http://nutrition.anamai.moph.go.th/ewt_news.php?nid=440, accessed 22 July, 2019.
World Health Organization. Tobacco free initiative. Policy recommendations for smoking cessation and treatment of tobacco dependence. Available from: http://www.who.int/tobacco/resources/ publications/tobacco_dependence/en.full, accessed 21 December, 2013.
Patent
Cramm NT, inventor. A device to simplify the conversion of bibliographic information into citation format. U.S. Patent no. 7 005 423. 13 September, 2005.
1.5 Peer Review Process
Manuscripts that pass the primary and secondary screening steps, outlined in the Manuscript Processing Flowchart, will be sent to 3 peer reviewers.
The journal operates a double-blind peer review process.
Should your manuscript be sent for peer review, you can expect to receive the comments from each peer reviewer, regardless of the outcome of the review (reject, minor revision, major revision, accept). The editors are responsible for making the final decision based on the reviewers’ comments.
If your manuscript is sent for peer review, we aim to obtain and send peer reviewers’ comments back to authors within six weeks. If you are recommended to revise your manuscript, you are expected to address each of the comments received, and return your revisions in a timely manner within the due date stipulated by the editorial office. Not doing so may result in further delays, or you may have to re-submit your manuscript as a new submission and begin the whole peer review process again.
Revisions to your manuscript must be underlined or shown in tracked changes. Authors must provide an explanation of the revisions in the form of a cover letter. The cover letter should show the reviewers’ comments and an explanation of your revision or rebuttal alongside, which may be in the form of a two-column table. A template Cover Letter is available for authors’ use.
Please also note that it is most common for manuscripts to be sent to reviewers more than one time. Moreover, if a reviewer declines to see a revised manuscript, we will find a new reviewer.
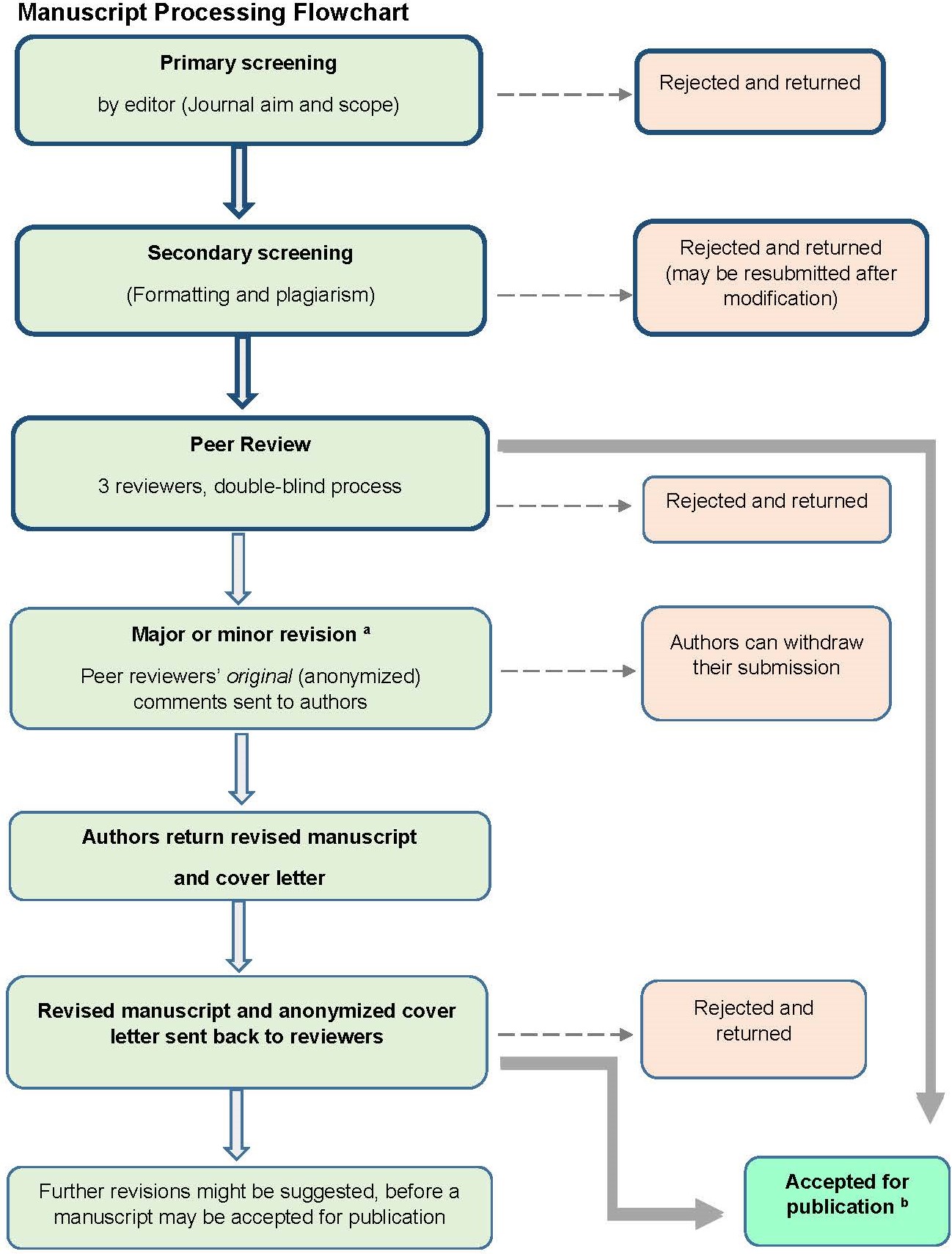
aA major revision needs to be returned to the reviewers; examples of points that warrant major revision: Inclusion of extra literature or theory, clarification of the methods, and time-consuming improvement of arguments and conclusions. A minor revision means that the manuscript is close to being publishable and should not need to be re-reviewed by peer reviewers; examples of points that warrant minor revision include deleting redundant material and tweaking arguments. More detailed information on our Manuscript Decision Making Criteria is available here.
bAfter acceptance for publication, proofs are prepared (formatted, checked for grammar and points that lack clarity). Any queries raised are noted on proofs which are then sent to the corresponding author, to allow a final check for any errors and improvement of any points that lack clarity.
1.6 Publication Ethics
The Thai Journal of Public Health operates in accordance with COPE (Committee of Publication Ethics) Core Practices (https://publicationethics.org/core-practices), and is committed to adhering to ethical publication practices.
Duties of Authors:
- Follow our author guidelines and use the manuscript templates.
- Submit original work
Plagiarism of other authors’ work and the authors’ own work is strictly unacceptable. When reporting the work of other authors, authors must use their own words and use appropriate citations. Authors are responsible for obtaining written permission to use any material (e.g., photograph, figure, etc.) that has been previously published in any medium (e.g., electronic, hard print, etc.). A Letter Template for Seeking Permission to Use Reproduced Material is available, which authors may use, if the information source does not provide their own. As part of the manuscript screening process, all submitted manuscripts will undergo a check for plagiarism. Any manuscript which does not pass this screening step will be returned to the author, along with the precise reasons for the return.
- Demonstrate that relevant codes of practice were followed
The authors must state ethical approval/clinical trial registry/animal ethics approval details in their manuscript. If applicable, trial registration numbers should be given, along with reference to laboratory standards or other codes of practice, where applicable.
• Original research that involved human subjects must have received approval from a human ethics review board for the conduct of research involving humans. The authors must state the protocol number(s) assigned by the ethical review board(s) and the full name(s) and affiliation(s) of the ethical review board(s) that were responsible for approving the protocol(s) described in their manuscript. The authors must also describe the procedure for obtaining informed consent which was followed during the study. Researchers who conduct studies with human volunteers must comply with the principles set out in the Declaration of Helsinki.
• Human research studies that test the effectiveness or safety of medical products, such as drugs or devices, or foods, food components or supplements, or medical procedures, are clinical trials and should be registered on a clinical trials registry such as ClinicalTrials.gov.
• Research that involved animals should have received the necessary approval from an animal ethics review board, and details (name of committee, reference numbers etc.) must be provided.
- Submit real and accurate research data, without fabrication or manipulation
- Confirm that the work has not been published, and is not under review, elsewhere
- Consent to THJPH publishing the work
- Declare author contributions
Every author of the manuscript must have made an academic contribution to the work. The International Committee of Medical Journal Editors http://www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html provides guidance on meeting the requirements for authorship. They recommend that authors have played a role in conceiving or designing the work, or collecting, analyzing or interpreting data; and contributed to the drafting or critical revision of the work; and approved the final version for publication; and agreed to take responsibility for the work in relation to its precision and integrity.
- Acknowledge contributors who do not qualify for authorship
Individuals who made any type of contribution to the work at any stage (i.e., from the time the idea was conceived up to and including manuscript preparation), but who do not meet the requirements for authorship, must be acknowledged. For example, they may be data collection assistants, laboratory assistants, individuals who facilitated access to the research setting, etc. These individuals should have agreed with any acknowledgement written about them.
- Disclose sources of funding
Disclose the source(s) of funding and declare whether or not the funder played a role in data analysis or interpretation. If there was no funding or no formally provided funding for the work, authors should state "The authors did not receive funding to carry out the work presented in this article." or "The work presented in this article was self-funded." If there was a source of funding the authors should write: "This research received funding support from [name of funding agency], [grant number]".
- Declare conflicts of interest
These are usually relationships, connections or affiliations that could have influenced the results or the interpretation of the results. If there are none, please write: “The authors have no conflicts of interest to declare”.
Duties of Reviewers:
- Only agree to review a manuscript which falls within the remit of their expertise
- Avoid breaching confidentiality
Reviewers must not: share a manuscript or any part of a manuscript with another person, assign another person to do the peer review, or breach confidentiality in any other way.
- Only proceed with the review if they remain unaware of the identity of the author(s) and are not coauthors
- Provide constructive and timely comments on the peer reviewer’s comment form, which objectively focus on intellectual content and not the reviewer’s personal or preferred style
- Maintain confidentiality by avoiding wording that could reveal personal identity
- If recommending the addition of references to support information, avoid manipulating authors’ citations
- Notify the editorial office immediately if plagiarism or other ethical concerns are suspected
- Declare any competing interests (personal, financial, intellectual or otherwise) to the editorial office
Duties of Editors:
- Evaluate manuscripts to determine if they meet the journal’s aim and scope, and facilitate the improvement of their intellectual content to maintain the quality of the journal’s publications
- Protect the anonymity of authors and reviewers, as part of maintaining a double-blind review process, and not exploit submissions for personal gain
- Reject any manuscripts which fail to remove plagiarism that is detected at any stage of the review process
- Reject any manuscripts which are found to have been previously published or are under consideration for publication elsewhere
- Retain the right to retract a published article, in the case that there is proven misconduct
- Declare all conflicts of interest in relation to authors and reviewers, or any activities which may influence manuscript decisions
- Contribute towards the goals and plans of the journal, and participate in public relations by positively informing their networks about the journal
Process for Dealing with Allegations of Misconduct:
Misconduct covers: a submitted or published manuscript that is suspected to be a duplication of previously published work, suspected plagiarism in a submitted or published manuscript, suspected fabricated data in a submitted or published manuscript, suspected ghost, guest or gift authorship, suspected undisclosed conflict of interest, a suspected ethical issue, and where a peer reviewer is suspected of taking an author’s idea or data. If any such allegations of misconduct were to arise, they would be dealt with as outlined in the COPE guidelines. The journal’s process of responding to whistleblowers would also follow the COPE guidelines. These guidelines can be viewed and downloaded: https://doi.org/10.24318/cope.2019.2.26.
1.7 Advertising and Marketing Policies
We do not accept adverts on the journal's website or Facebook Fan Page.
1.8 Appeals
If an author suspects that the editorial team has made a mistake or he/she does not agree with the editor’s final recommendation, the author may make an appeal. In which case, the author should write an appeals letter and send it directly to the Editor. The appeals letter should state the author’s case, including reasons why he/she believes that the editor made an erroneous decision. All appeal letters will be considered and discussed by at least three members of the editorial team, including the Editor. A decision will be taken as to whether the author’s manuscript should be re-reviewed or otherwise.
Copyright Notice
The copyright of all articles published in the Thai Journal of Public Health are classified under the Creative Commons License CC-BY-ND. Published articles can be downloaded and redistributed providing that the work is correctly cited, and its content can be used commercially.
Privacy Statement
The names and email addresses entered in this journal site will be used exclusively for the stated purposes of this journal and will not be made available for any other purpose or to any other party.
